Teflon Is Forever
For decades, DuPont has sold the answer to crud, gunk, and grime. What the company didn’t advertise was that its nonstick wonder sticks—to us.
Congresswoman Pat Schroeder was scrambling eggs, one day back in 1984, when she coined one of the most durable political metaphors of our time. Her 1984 description of Ronald Reagan as “the Teflon President” became instant vernacular, attaching itself to everyone from “Teflon Tony” Blair to “Teflon Don” John Gotti.
It is all the more ironic, then, that our favorite metaphor for bad press that won’t stick comes from a product whose toxic legacy will stick around forever. Teflon, it turns out, gets its nonstick properties from a toxic, nearly indestructible chemical called pfoa, or perfluorooctanoic acid. Used in thousands of products from cookware to kids’ pajamas to takeout coffee cups, pfoa is a likely human carcinogen, according to a science panel commissioned by the Environmental Protection Agency. It shows up in dolphins off the Florida coast and polar bears in the Arctic; it is present, according to a range of studies, in the bloodstream of almost every American—and even in newborns (where it may be associated with decreased birth weight and head circumference). The nonprofit watchdog organization Environmental Working Group (ewg) calls pfoa and its close chemical relatives “the most persistent synthetic chemicals known to man.” And although DuPont, the nation’s sole Teflon manufacturer, likes to chirp that its product makes “cleanup a breeze,” it is now becoming apparent that cleansing ourselves of pfoa is nearly impossible.
DuPont has always known more about Teflon than it let on. Two years ago the epa fined the company $16.5 million—the largest administrative fine in the agency’s history—for covering up decades’ worth of studies indicating that pfoacould cause health problems such as cancer, birth defects, and liver damage. The company has faced a barrage of lawsuits and embarrassing studies as well as an ongoing criminal probe from the Department of Justice over its failure to report health problems among Teflon workers. One lawsuit accuses DuPont of fouling drinking water systems and contaminating its employees with pfoa. Yet it is still manufacturing and using pfoa, and unless the epa chooses to ban the chemical, DuPont will keep making it, unhindered, until 2015.
The Teflon era began in 1938, when a DuPont chemist experimenting with refrigerants stumbled upon what would turn out to be, as the company later boasted, “one of the world’s slipperiest substances.” DuPont registered the Teflon trademark in 1944, and the coating was soon put to work in the Manhattan Project’s A-bomb effort. But like other wartime innovations, such as nylon and pesticides, Teflon found its true calling on the home front. By the 1960s, DuPont was producing Teflon for cookware and advertising it as “a housewife’s best friend.” Today, DuPont’s annual worldwide revenues from Teflon and other products made with pfoa as a processing agent account for a full $1 billion of the company’s total revenues of $29 billion.
Teflon is not actually the brand name of a pan; it’s the name of the slippery stuff that DuPont sells to other companies. Marketers deploy the trademark as a near-mystic incantation, a mantra for warding off filth: Clorox Toilet Bowl Cleaner With Teflon® Surface Protector, Dockers Stain Defender™ With Teflon®, Blue Dolphin Sleep ‘N Play layette set “protected with Teflon fabric protector.” In one TV spot, an infant cries until Dad sets him down on a Stainmaster (with Advanced Teflon® Repel System) carpet, where baby, improbably, falls into blissful slumber.
Breathing in dust from Teflon-treated rugs or upholstery as they wear down is one way we may be ingesting pfoa. Food is another: Pizza-slice paper, microwave-popcorn bags, ice cream cartons, and other food packages are often lined with Zonyl, another DuPont brand. Technically, Zonyl does not contain pfoa, but it is made with fluorotelomer chemicals that break down into pfoa. Regardless of how it gets into our bodies, once there, pfoa stays—quietly accumulating in our tissues, for a lifetime.
Teflon is not the only nonstick, non-stain brand that has turned out to be stickier than advertised. Scotchgard and Gore-Tex, to name just two, are also made with pfoa or other perfluorochemicals (pfcs). Last year the epa hit the 3M corporation, maker of Scotchgard, with a $1.5 million penalty for failing to report pfoa and pfc health data. Chemicals similar to pfoa have recently turned up in water supplies of suburban Minneapolis and St. Paul, near 3M facilities.
Unlike DuPont, though, 3M no longer sells pfoa: In the late 1990s, when testing blood samples for a health study, the company found pfoa even in the “clean” samples from various U.S. blood banks that it had planned to use as controls. “They realized they were contaminating the entire population,” says Richard Wiles, the Environmental Working Group’s executive director. In 2000, 3M announced that it was discontinuing pfoa production.
When 3M got out, DuPont, which until then had bought its pfoa from 3M, jumped in. Now the company’s bottom line depends on whether its product’s mythic reputation—Teflon’s own Teflon—remains intact.
So far, it seems to be holding. Nonstick pots and pans account for 70 percent of all cookware sold. “Amazingly enough, all the publicity has had no impact on sales,” says Hugh Rushing, executive vice president of the Cookware Manufacturers’ Association. “People read so much about the supposed dangers in the environment that they get a tin ear about it”—though sales of cast-iron skillets, touted as a safer alternative, have doubled in the last five years, in large part because of “the Teflon issue,” according to cast-iron manufacturer Lodge.
In fact, nonstick pans are not a major source of exposure to pfoa, because almost all of the chemical is burned off during manufacture. Still, when overheated, Teflon cookware can release trace amounts of pfoa and 14 other gases and particles, including some proven toxins and carcinogens, according to the Environmental Working Group’s review of 16 research studies over some 50 years. At 500 degrees, Teflon fumes can kill birds; at 660, they can cause the flulike “polymer fume fever” in humans. Even at normal cooking temperatures, two of four brands of frying pans tested in a study cosponsored by DuPont gave off trace amounts of gaseous pfoa and other perfluorated chemicals.
A $5 billion multistate class-action lawsuit representing millions of Teflon cookware owners alleges that DuPont has known for years that its coatings could turn toxic at temperatures commonly reached on the stove, but failed to tell consumers. DuPont’s website recommends not heating Teflon above 500 degrees (so it doesn’t “discolor or lose its nonstick quality”) and advises that when overheated, “nonstick cookware can emit fumes that may be harmful to birds, as can any type of cookware preheated with cooking oil, fats, margarine and butter.” But who knows how hot a pan gets, and who looks out for birds before fixing dinner? Even while researching this story, I left a nonstick skillet on the stove. The fumes smelled like fried computer, and I vowed not to do it again. But I also decided to go with the hazardous-waste flow, figuring, “We’re all toxic dumps anyway.” (ewg studies have found a “body burden” of 455 industrial pollutants, pesticides, and other chemicals in the bodies of ordinary Americans.) With toxic substances unavoidable, or at least key to convenience, we run our own self-interested cost-benefit analyses. I throw out the Teflon-coated Claiborne pants my mother-in-law sent my son, but I let him play on swing sets made of arsenic-treated wood because I don’t want to face a tantrum.
Still, consumers of Teflon pans and pants (not to mention the mascara, dental floss, and other personal care products made slippery with a touch of Tef) have it relatively safe. The people who make the stuff, and who live near the plants, face far worse dangers. The granddaddy of trouble plants—and the one inspiring a range of lawsuits—is DuPont’s plant near Parkersburg, West Virginia. Residents there have sued DuPont for polluting their drinking water with pfoa, and in March 2005, DuPont settled the case for $107 million. If an independent science panel finds links between pfoa and various health problems, DuPont will have to pay up to an additional $235 million to monitor the health of 70,000 people for years to come. Meanwhile, as part of the court order, the company is supplying the entire population of one nearby town with bottled drinking water.
The epa’s $16.5 million fine against DuPont for concealing evidence of health risks traces back to the same Parkersburg plant. According to the epa, workers were reporting health problems there for years, including birth defects in their children; as far back as 1981, DuPont scientists knew that pfoa could cross the placenta and thus contaminate fetuses. DuPont also knew that some of its workers’ babies had been born with eye defects similar to those 3M had just then reported in rats exposed to pfoa. At that point, rather than risk finding more evidence, DuPont terminated its study and didn’t report the troubling data to the epa as required by law. “Our interpretation of the reporting requirements differed from the agency’s,” the company explained in 2005.
Today, DuPont remains adamant that pfoa—whether in pots, pants, or drinking water—is no threat. The epa may say studies show unequivocally that in “laboratory animals exposed to high doses, pfoa causes liver cancer, reduced birth weight, immune suppression and developmental problems,” but DuPont’s website quotes Dr. Samuel M. Cohen of the University of Nebraska Medical Center, who says, “We can be confident that pfoa does not pose a cancer risk to humans at the low levels found in the general population.” But, notes Robert Bilott, one of the lead attorneys in the Parkersburg suit, “the general population isn’t drinking it. And they have five parts per billion in their blood. Near the West Virginia plant, it’s in the hundreds of parts per billion; and in the elderly and in children, several thousand parts per billion.”
DuPont is hardly unique in trying to cast unflattering data as incomplete or uncertain. As epidemiologist David Michaels wrote in a 2005 essay in Scientific American titled “Doubt Is Their Product,” many corporations have followed the tobacco (and more recently, global warming) model of insisting that the scientific jury is still out, “no matter how powerful the evidence.” Michaels took his title from a 1969 memo written by an executive for cigarette maker Brown & Williamson: “Doubt is our product since it is the best means of competing with the ‘body of fact’ that exists in the mind of the general public.” Even the indoor tanning industry, notes Michaels, “has been hard at work disparaging studies that have linked ultraviolet exposure with skin cancer.”
Chemical companies caught a break with the passage of the 1976 Toxic Substances Control Act (which they helped write), a measure so weak it doesn’t require industrial chemicals to be tested for toxicity. Only toxic effects, often found after a product has become ubiquitous in the environment and in people’s bodies, must be reported—and even that rule, as DuPont discovered, can be broken with only a minor hit to profits.
In the case of pfoa, it was left to the epa to finally investigate the risk to public health. That assessment, begun in 2000, is expected to go on for years. If pfoa is determined to be a proven (not merely likely) carcinogen, says agency spokeswoman Enesta Jones, “this chemical could be banned.” It would be one of the epa‘s very few outright bans since 1996, when it proscribed ozone-depleting chlorofluorocarbons. DuPont was the world’s biggest producer of those too.
For now, DuPont is subject only to the epa‘s voluntary “stewardship” program, under which it has agreed to reduce pfoa emissions from products and factories by 95 percent by 2010 and 100 percent by 2015. DuPont says it is likely to meet those deadlines: In February, the company announced it had found a new technology that reduces by 97 percent the pfoa used in making Teflon and other coatings, and it has vowed to “eliminate the need to make, buy or use pfoa by 2015.”
“It’s interesting how DuPont says they’re going to eliminate the ‘need’ to make, buy, or use pfoa,” says Rick Abraham, an environmental consultant for the United Steelworkers, which represents workers at DuPont’s plants. “It’s a self-imposed need. They need it to make money. Are they going to stockpile it, make as much as they can by 2015? Given DuPont’s history, that’s very possible. They need to make public a time frame for annual production and have it subject to third-party verification.” DuPont spokesman Dan Turner responds, “We’re going to eliminate it, period.” As for time frames, he says, “I can’t get into specifics. I can only say we’re moving as quickly as the technology allows.”
Meanwhile, DuPont has been applying a protective layer of PR to the problem. Last year, caught in a flurry of bad publicity about fines and lawsuits, the company took out full-page newspaper ads. One stated, “Teflon® Non-Stick Coating is Safe.” And, as if to flip the bird at workers’ complaints, it ran an ad in Working Woman showing a female factory worker and declaring: “DuPont employees use their skills and talents to make lives better, safer and healthier.” This year, DuPont plans to advertise its pfoa-lowering measures only in trade publications, perhaps because it’s tricky to boast of reduced pfoa while also maintaining that the chemical is harmless. “No one is better than DuPont at greenwashing,” says Joe Drexler of the Steelworkers’ DuPont Accountability Project.
Possibly. Recall DuPont’s 1990 “Ode to Joy” commercial, in which seals clapped, penguins chirped, and whales leapt to honor DuPont for using double-hull tankers to “safeguard the environment.” The seals evidently didn’t realize that a law passed after the 1989 Exxon Valdez oil spill required double-hull tankers. The penguins probably didn’t connect the ice melting under their flippers with DuPont’s chlorofluorocarbons either. The company fought against regulating them right up until they were banned.
It is in such ads that corporate fantasies and our individual ones meet and agree to ignore unpleasantries. Corporations lie to us, sure, but we make it easy for them with the little lies we tell ourselves. Especially when it comes to our everyday conveniences, it’s easier to accept the company line that there is no risk than it is to accept that authorities won’t necessarily protect us from risk. Jim Rowe, president of the union local at DuPont’s Chambers Works plants in New Jersey, told me that despite the science about birth defects among DuPont employees, many of his coworkers have convinced themselves that there’s nothing to worry about: “When we took blood tests and interviewed them, they said they were told ‘pfoa‘s not a problem—it’s even in polar bears.'” Precisely. And even if DuPont (and companies that make pfoa in Europe and Asia) stopped producing and using the chemical tomorrow, the millions of pounds of it already on earth would remain in the environment and in our bodies “forever,” says the ewg‘s Wiles. “By that we mean infinity.”
Denial, avoidance, and magical thinking aren’t new. Like Teflon, they’re barriers that keep unpleasant things at bay, and like Teflon, they’re entrenched deep inside us.
Tags:teflon ptfe,teflon
PTFE WIRE APPLICATIONS
PTFE APPLICATIONS
PTFE’s significant chemical, temperature, moisture, and electrical resistances make it an ideal material whenever products, tools, and components need to be durable and reliable in even the most strenuous applications. On top of this, teflon coated wire boasts unique low- temperature durability and fire resistance that make it a good choice for a constantly growing list of products, components, and applications.
These qualities have allowed PWI to provide insulated PTFE wire for an extensive range of high tech industries across the country. While PTFE coated wire is often referred to by it's most popular brand-name, Phoenix Wire has made it our specialty to start where most other companies stop, and provide PTFE insulated wire not only in all standard sizes, but in a range of micro and miniature sizes that continue to enable innovation in even the most advanced industries.
Coated Wire for Medical Applications
PTFE coated wires provide ideal coverage and protection when medical devices require a smooth coatings that’s thin, smooth, precise, chemically inert, and capable of withstanding a wide variety of conditions. It’s non-flaking finish makes it a coating of choice when finish quality is paramount for both aesthetics and regulatory specifications. At PWI, our specialty is insulating the kind of micro PTFE coated wire that is frequently used in medical instrumentation, implants, and other devices.
PTFE Insulated Wire for Automotive Applications
The electrical demands placed on wiring in automotive applications continue to demand more. With the presence of corrosive chemicals, extreme temperatures, and friction - durable automotive wiring is relied on for a growing list of applications including air conditioning systems, navigation,power steering,battery applications, heated seats, and more.
With micro miniature teflon coated automotive wires, PWI gives automotive manufacturers the ability to continually innovate with wiring that meets their exact performance specifications, no matter how limited they may be on space.
PTFE Coated Cable and Wire for Oil and Gas Applications
One of the greatest engineering challenges for the Oil and Gas industry is the ability to protect important instrumentation from temperature extremes, corrosive chemicals, and pressure. Fortunately, the low footprint and outstanding qualities of PTFE have allowed PWI to address the oil and gas industry’s complex wiring needs for drilling operations and instrumentation in even the toughest conditions.
On top of insulation, teflon coated wires not only offer the electrical, temperature, and corrosion resistance needed by the gas and oil industries, but they also provide essential protection against the gas diffusion, pressure, and corrosion typically encountered in downhole drilling.
PTFE Coated Wires for General Electrical Applications
As one of the best known insulators, PTFE is frequently used in electrical components around the world for its ability to insulate to 500 volts per mil with unyielding reliability in even the most strenuous applications.
From mobile devices to advanced, high-tech machinery -- PTFE coated wires can be found in virtually every industry. It is often used as wire and cable wrap, as a separator on the conductive surfaces in capacitors, and in a limitless range of electrical applications where components are expected to withstand the elements.
With modern electronic manufacturers continually creating smaller, more portable advanced electronics, a cost efficient and reliable source for teflon coated wire has never been more important.
Other Applications for PTFE Insulated Wire
The qualities of PTFE lend our products to an almost infinite list of uses. Other industries and applications PWI works with include retail, computers, veterinary medicine, the food industry, communications, the art community, robotics, marine sciences, space exploration, bioengineering, chemical sciences, and more.
Tags:teflon,teflon ptfe,teflon wire
PFA and TFM PTFE Excellent Material Choice for Bellows and Diaphragms
6 Key Reasons PEEK Works Well for High-Performance Bushings
The combined strength, thermal, and chemical properties of polyetheretherketone make it an excellent choice for high temperature, environments in need of bushings. Add to that its ability to serve as an excellent replacement for metal bushings, and its ability to have its properties further enhanced by additives like glass and carbon, and PEEK quickly stands out as a top choice for high-performance polymer bushings.
Tags:PEEK,High-Performance Bushings
Five Ways that PTFE Rotary Seals Differ from ElaomstericSeals
The PTFE Rotary Seal Difference
PTFE rotary seals are often the answer when elastomeric seals just can’t handle the demands. In this article we are going to look at just five ways that PTFE seals differ in performance and behavior from elastomeric seals.
Here are some additional blog posts from the Advanced EMC Technologies Blog:
•Out of Whack: Eccentricity and Runout in PTFE Rotary Seals
•PTFE Rotary Lip Seals - 6 Feature Competitors Don't Want You to Know!
•Rotary Seals for Dummies: Four Questions about Shaft Surfaces for PTFE Rotary Seals
Low Friction
Because of the incredibly low coefficient of friction that PTFE has, it can be used in applications where lubricant cannot be used. This is referred to as “dry running,” and PTFE seals excel in these types of applications where elastomeric seals fail.
Speed
Because of the low friction and excellent wear capabilities of PTFE, most PTFE seals can withstand running speeds of up to 5,900 feet per minute, or 30 m/s. This makes them ideal for speed-intensive applications where reliable sealing is vital.
Chemical Compatibility and FDA Approval
PTFE is known for its incredible compatibility with a variety of chemicals, which sets it apart from the elastomeric materials typically used in sealing applications. Many PTFE compounds already FDA approval and are commonly used in pharmaceutical, food, and dairy applications
.
Operating Temperatures
Another benefit of PTFE rotary seals over traditional elastomeric rotary seals is the temperature range over which they can operate. Most PTFE seals can perform in the cryogenic temperatures all the way down to -95°F up and up to extremely high temperatures of 480°F.
Relationship between Speed and Friction
The hydrodynamic film all the separates the seal lip from the movie.How much friction exists between the seal and the sealing surface is a function of the thickness of the hydrodynamic film. The film pulled into the gap between the seal and the surface by viscous drag. When the shaft is at rest, this layer will be at its minimum thickness and a certain amount of torque will be required to overcome the initial resistance to motion. Friction decreases as the velocity increases up to a point; after that speed is reached, friction will again begin to rise and the seal may begin to experience wear. However, PTFE has a very low coefficient of friction to begin with, and may often be an exception to this rule.
PTFE Seals Alternative
The next time you are choosing a dynamic seal for an application that involves high speeds, extreme temperatures, a need for low friction, FDA approval, or chemical resistance, don't forget to look intoPTFE seals as an alternative to the traditional elastomeric dynamic seals.
For more detailed information on PTFE Rotary Shaft Seals download Advanced EMC Technologies resource guide.
Tags:rotary seal,ElaomstericSeals,ptfe
Custom Spring Energized PTFE Seals for Medical Devices
The medical device industry faces continually evolving challenges when it comes to finding the right sealing solutions for new and improved designs. Issues such as sterilization, wide ranges of expected pressure, potentially aggressive environments, and FDA and USP approval make the design and specification process quite challenging. In this article, we are going to look at custom spring energized PTFE seals as a potential solution for sealing challenges in the medical industry.
Why PTFE Seals?
PTFE is a popular choice for spring energized seals for medical applications for several reasons. One is the fact that certain grades of PTFE have been approved by the FDA as USP Class VImaterials. It is resistant to a variety of aggressive chemicals, has extremely low friction, and retains its key characteristics – including strength – over a wide range of temperatures and pressures. It can be sterilized using methods such as steam and EtO (ethylene oxide), and is both hydrophobic and oleophobic.
Why Spring Energized Seals?
As you probably already know, spring energized seals are able to achieve a seal at low pressures because the spring applies outward pressure to the lip of the seal against the shaft or bore. As pressures increase, the pressure itself takes over from the energizing spring and achieves a tight seal. The result is an effective sealing solution.
Where Are Energized PTFE Seals Used in the Medical Device Industry?
PTFE seals are a common sight in the medical device industry, found in everything from dialysis equipment and infusion pumps to oxygen therapy, implanted electronic devices, trochars, and IV systems. Spring-energized PTFE seals are used in medical instruments, drug delivery systems, and orthopedic applications, just to name a few.
Custom Seals
Custom seal designs are available to meet the complex needs of the medical device industry. This includes custom engineering of the polymer (including fillers), unusual sizes or geometries, special spring materials, and more. In addition, PTFE lends itself to manufacturing processes such as machining that offer a high degree of accuracy and precision.
Conclusion
PTFE seals are popular in the medical device industry for a variety of reasons, including their low friction, chemical resistance, and excellent performance in a variety of pressure, temperature, and speed situations. Spring-energized PTFE seals provide a reliable sealing solution that is effective even in low-pressure environments. Even if an off-the-shelf energized PTFE seal won’t meet your needs, you can look into a custom-designed energized Teflon seal tailored to your requirements and specifications.
Tags:seal,custom,ptfe,medical
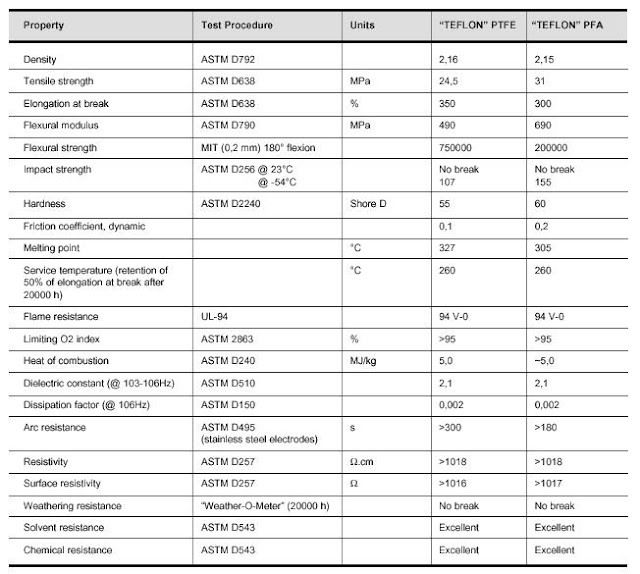
Comparison Between PTFE and PFA Processing
For a number of years fluoropolymers have played a significant role in the chemical and similar industries to protect plants and equipment against chemical attack by a broad range of aggressive media. This is because they offer substantially better chemical resistance and thermal stability than other plastics or elastomeric materials.
Following the development of PTFE, the introduction of melt-processable fluorinated ethylene-propylene (FEP) in 1960 opened up entirely new application areas. PFA, a perfluoro-alkoxy polymer which has been in successful use for 20 years as a lining material, is now a thermoplastic successor to PTFE, with equivalent thermal and chemical resistance and superior properties with respect to processability, translucency, permeation resistance and mechanical strength.
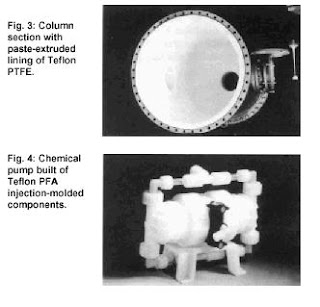
In the chemical industry, both fluoropolymers - PTFE and PFA - are used mainly in the form of linings (fig. 1, 2). For simple shapes, such as pipes, bends, T-pieces or reduction joints, PTFE is generally used; it is applied by means of paste extrusion, ram extrusion or tape wind-ing (fig. 3). In these processes a pre-form is made of the PTFE; this is then sintered and inserted into the metal workpiece. Using PTFE for lining of metal parts of complicated shape, such as valves and pumps, is more difficult. Isostatic molding is then the preferred method. In this PTFE powder is filled into the space created between the metal work-piece and a rubber bag which is specially made to fit into the shape of the area to be lined. The powder is pre-compressed, then cold-pressed into the desired shape. Finally, the rubber bag is removed and the lined part is sintered in an oven at over 360?C (680?F).
PFA, a thermoplastic material with a well-defined melting point, can be processed by means of transfer molding or injection molding. The granulate is melted in a melt pot or in the extruder and then forced into the hot tool by a hydraulic press.
This method enables very precise wall-thicknesses to be achieved, with tolerances of ? 0,5 mm, even at tight radii and in undercuts. Practically no mechanical finishing is needed, except to remove the sprue and to smooth the mating faces of flanges.
When using isostatic molding, however, a considerable amount of mechanical finishing is needed - depending on the degree of complication of the shape to be filled - to achieve the desired dimensions with precision.
The evenness of the wall-thickness may vary more, especially in the case of more complicated shapes such as valve housings.